Operability of glioblastomas: “sins of action” versus “sins of non-action”
Transcript of Operability of glioblastomas: “sins of action” versus “sins of non-action”

ORIGINAL ARTICLE
Operability of glioblastomas: ‘‘sins of action’’ versus‘‘sins of non-action’’
Paolo Ferroli • Marco Schiariti • Gaetano Finocchiaro • Andrea Salmaggi •
Melina Castiglione • Francesco Acerbi • Giovanni Tringali • Mariangela Farinotti •
Morgan Broggi • Cordella Roberto • Elio Maccagnano • Giovanni Broggi
Received: 20 September 2012 / Accepted: 22 February 2013 / Published online: 12 March 2013
� Springer-Verlag Italia 2013
Abstract Despite prognosis of glioblastomas is still poor,
mounting evidence suggests that more extensive surgical
resections are associated with longer life expectancy.
However, the surgical indications, at present, are far from
uniform and the concept of operability is extremely sur-
geon-dependant. The results of glioblastoma resection in
104 patients operated on between March 2005 and April
2011 were reviewed with the aim to shed some light on the
limits between ‘sins of action’ (operating upon complex
tumors causing a permanent severe deficit) and ‘sins of
non-action’ (considering inoperable tumors that can be
resected with good results). Fifty-five patients (54.4 %)
(Group 1) presented with a ‘disputable’ surgical indication
because of one or more of the following clinico-radiolog-
ical aspects: involvement of motor and language areas
(39.4 %), deep location (7.7 %), corpus callosum infiltra-
tion (13.4 %), or major vessels encasement (8.6 %). Forty-
six (42.5 %) patients (Group 2) presented with an ‘indis-
putable’ surgical indication (readily accessible tumors in
non-eloquent areas). Overall mortality was 2.9 %. The
mean overall survival was 19.8 months and not signifi-
cantly different in the two Groups (20.4 Group 2 and 19.5
months for Group 1; p = 0.7). Patients with GTR and\72
years had a longer survival (p = 0.004 and 0.03, respec-
tively). Seventy patients (69.3 %) showed an uneventful
post-operative course, without statistical significance dif-
ference between Group 1 and 2. The gross total removal of
glioblastoma with many complexities (Group 1) was found
to be feasible with acceptable mortality, morbidity and
long-term survival rates.
Keywords Operability � Glioblastoma � Quality of life �Survival � Neurosurgery
Introduction
Despite prognosis of glioblastomas is still poor, mounting
evidence suggests better outcomes (prolonged post-surgical
life expectancy) after extensive surgical resection [1–4].
For this reason in dedicated brain cancer centers, a
‘‘maximal safe resection’’ of all tumors is the standard of
care. However, patients affected by complex tumors where
surgery poses the threat of inflicting new invalidating
neurological deficits still receive very different opinions
about surgical indication and many surgeons still apply the
label of ‘‘inoperability’’ to some cases. The most common
reasons for that are: multifocality; contralateral extension;
infiltration of supposed eloquent areas; encasement of
major cerebral vessels, and deep location. In our series,
none of these features were considered as an absolute
contraindication and the envelope of surgical indication
was pushed far beyond conventional limits. In this study,
we present the results of glioblastoma resection in 104
P. Ferroli (&) � M. Schiariti � M. Castiglione � F. Acerbi �G. Tringali � M. Broggi � C. Roberto � G. Broggi
Department of Neurosurgery, Fondazione Istituto Neurologico
Carlo Besta, Via Celoria 11, 20133 Milan, Italy
e-mail: [email protected]
G. Finocchiaro � A. Salmaggi
Department of Neuro-oncology, Fondazione Istituto Neurologico
Carlo Besta, Via Celoria 11, 20133 Milan, Italy
M. Farinotti
Department of Neuro-epidemiology, Fondazione Istituto
Neurologico Carlo Besta, Via Celoria 11, 20133 Milan, Italy
E. Maccagnano
Department of Neuro-radiology, Fondazione Istituto
Neurologico Carlo Besta, Via Celoria 11, 20133 Milan, Italy
123
Neurol Sci (2013) 34:2107–2116
DOI 10.1007/s10072-013-1345-5

patients with the specific aim of shining some much light
on the question of ‘limits’ in the critical debate between
‘‘sins of action’’ (i.e. surgical procedures done on the
complex GBM patient that cause severe, permanent neu-
rological deterioration) and ‘‘sins of non-action’’ (i.e. sur-
geries not performed on ‘inoperable’ grounds that could
have resulted in a resection with positive outcomes).
Patients and methods
Between March 2005 and April 2011, 104 patients affected
by GBM were operated upon at the Department of Neu-
rosurgery of the C. Besta Institute of Milan by a single
surgeon (PF) with the philosophy of ‘‘maximal safe
resection’’ in all cases. The reasons to study the work of
this single surgeon, retrospectively, were to avoid:
1. any bias in selecting patients arising from different
attitudes and experiences;
2. any bias related to different surgical techniques.
All demographic and clinical features were obtained
from our hospital records. This retrospective study received
the approval of our Institutional Ethical Committee and the
condition of an ‘‘Informed Consent’’ was obtained in all of
the patients that were studied.
We made no attempt to recognize such factors as: age,
cardiopathies, diabetes, obesity, motor dysfunctions, and
speech disturbances as ‘absolute contraindications’ when it
was obvious that a surgical intervention was necessary to
prolong life, provided that the patient willingly accepted
the risk of possible complications. A Karnofsky score of
less than 60 was also bypassed as a contraindication if it
was related to an acute intracranial hypertension syndrome
that was likely to improve after surgery.
The intensity of motor deficit was rated using the fol-
lowing standardized motor scale: 0, no deficiency; 1, mild
deficiency (patient can use his or her limbs almost nor-
mally—i.e. walking is possible, but there is impairment of
fine movements of the upper limbs); 2, moderate deficiency
(movement is possible with the help of the examiner); and
3, severe deficiency (no spontaneous movement against
gravity) [5]; language disturbances were graded as mild,
moderate or severe according to NIHSS (National Institutes
of Health Stroke Scale) [6]. All patients were selected for
surgery after a complete contrast-enhanced brain MRI
examination. Functional MRI was used for studying
hemispheric dominance, language function and motor
function. DTI was used to track cortico-spinal pathways
and dorsal and ventral language pathways [7]. All data
were available for intraoperative navigation (StealthSta-
tion, Medtronic Inc.). Proton spectroscopy was used to help
making a differential diagnosis, when needed. Angio-RMs
or angio-TCs were acquired in the case of complex vas-
cular anatomical relationship among tumors, veins and
arteries.
The following clinico-radiological aspects were con-
sidered to increase surgical complexity:
1. Contrast enhancement/necrotic component involving
the primary motor cortex (clinically and fMR demon-
strated).
2. Contrast enhancement/necrotic component involving
the cortico-spinal tract (clinically and DTI demon-
strated).
3. Contrast enhancement/necrotic component involving
cortical language areas (clinically and fMR demon-
strated).
4. Contrast enhancement/necrotic component involving
language subcortical pathways (clinically and DTI
demonstrated).
5. Contrast enhancement/necrotic component involving
basal ganglia/thalamus/brainstem.
6. Contrast enhancement/necrotic component with con-
tralateral extension through midline connections.
7. Major cerebral vessels (ICA, ACA, MCA, PCA)
within the contrast-enhanced/necrotic component.
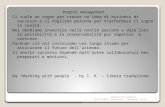
Some significant examples of these complexities are
illustrated in Fig. 1. The pre-operative characteristics of the
patients are summarized in Tables 1 and 2. This study
involved GBMs that were categorized as: primitive
(66.6 %); secondary (14.2 %); and recurrent (19.2 %). The
mean tumor diameter was 47.5 mm (median 47.8 mm;
range 18.1–80.2 mm). Patients were divided into 2 Groups
(Table 1): Group 1 (complex cases) consisted of 58
patients (55.7 %) in which the patients presented with one
or more of the above mentioned clinico-radiological
aspects that were considered to increase surgical com-
plexity. Group 2 consisted of the remaining 46 patients
(44.2 %) with readily accessible tumors in non-eloquent
areas.
Post-operative treatment and imaging
All primitive and secondary GBMs received combined
radio and chemotherapy (RCT) according to the Stupp
protocol (Temozolomide TMZ 75 mg/m2 1 h before stan-
dard focal radiotherapy (60 Gy) followed by additional
monthly cycle of TMZ 150/200 mg/m2 5/28) [8] and
completed the treatments. Fotemustine, procarbazine,
bevacizumab, irinotecan and cisplatin administration were
used as second line treatment for 35 patients.
Extent of resection based on residual tumor volume was
determined in all cases within 24–72 h after surgery by
comparison between preoperative and early post-operative
2108 Neurol Sci (2013) 34:2107–2116
123

T1-weighted contrast-enhanced MRI (within 72 h of sur-
gery) by a dedicated neuroradiologist (E.M.) following a
volumetric analysis. According to the classification system
reported by Berger [9] resection was considered ‘total’ in
cases where there was no residual contrast enhancement;
‘subtotal’ in cases with less than 10 cm3 of tumor residue;
and ‘partial’ in cases with more than 10 cm3 of tumor
residue.
Outcome measures and statistical analysis
Short term surgical outcome and post-operative
complications
The clinical and neurological status of the patient was
evaluated immediately after surgery with the intention of
revealing any possible new undesired neurological prob-
lem. The same grading scales used pre-operatively were
repeated in the immediate post-operative follow-up.
The results were graded as illustrated in Table 3. In
addition, any complication was recorded following Land-
riel-Ibanez classification [10].
Long-term results
Long-term results in terms of quality of life were assessed
by clinical follow-up and periodic phone survey. A neu-
rological examination was carried out. The QoL of these
patients was classified in four decreasing levels according
to Heros classification [11]. The ultimate data collection
and retrospective analysis of outcome were carried out by
an independent reviewer (MC). Survival rate came from
the Institutional Cancer Registry.
Statistical analysis
Epidemiological and clinical data were recorded. The inter-
group morbidity was compared with the Chi square test. Sur-
vival as a function of time was plotted using the Kaplan–Meier
method. The impact on survival rate of the following param-
eters: involvement of thalamus/basal ganglia/brainstem,
involvement of corpus callosum, major brain arteries encase-
ment, presence/absence of motor deficit, presence/absence of
language deficits, KPS score \or C80, tumor size \or C4
cm, age and tumor resection were evaluated. Univariate com-
parison of parameters was performed by log-rank analysis.
Fig. 1 Some significant examples of patients with a disputable surgical indication that were operated upon with good post-operative results
Neurol Sci (2013) 34:2107–2116 2109
123

Table 1 Characteristics of 101
patients [3 patients (2.88 %) in
Group 1 died in the first post-
operative week]: radiological
features and site, presenting
symptoms, pre-operative
examination results
sl slight deficit, mo moderate
deficit, se severe deficit
(according to NIHSS
classification)
Characteristics (no of patients)
No of cases and
radiological features
Presenting symptoms Pre-op exam results
Group 1 (55)
Motor and language areas (41) Seizures (13) Normal (10)
Reduction of ideation (2) Cognitive impairment (5)
Motor circuits involvement (17) Short term memory
impairment (3)
Motor deficits (sl = 4; mo = 12;
se = 2)
Language areas (13) Headache (6)
Both (11) Headache and vomiting (4) Speech deficits (sl = 9; mo = 12;
se = 1)Motor disturbances (6)
Language disturbances (7)
Deep location only (8) Headache (3) Normal (3)
Extrapyramidal symptoms (2) Cognitive impairment (2)
Thalamus (3) Motor disturbances (2) Motor deficits (sl = 0; mo = 2;
se = 3)
Brainstem (1) visual field defect (1)
Vertigo and dizziness (1)
Basal ganglia (4)
Corpus callosum (14) Seizures (2) Normal (5)
Corpus callosum only (4) Headache (4) Cognitive impairment (5)
Motor disturbances (3) Motor deficits (sl = 2; mo = 2;
se = 1)
Speech deficits (sl = 1; mo = 1;
se = 0)
Anterior (4) Language disturbances (1) visual field defect (2)
Median (1) Visual field defect (1)
Posterior (1)
Frontomesial (4) Ataxia (1)
Parietomesial (4)
Behavioral anomalies (2)
Vessels encasement (9) Headache (2) Normal (3)
Vessels encasement only (2) Motor disturbances (3) Motor deficits (sl = 2; mo = 0;
se = 2)
Speech deficits (sl = 3; mo = 2;
se = 1)
Carotid artery (1) Language disturbances (4)
Anterior cerebral artery (1)
Middle cerebral artery (6)
Posterior cerebral artery (1)
Group 2 (46) Seizures (14) Normal (32)
Frontal (19) Headache (13) Motor deficits (sl = 8; mo = 1;
se = 0)
Reduction of ideation (4) Speech deficits (sl = 2; mo = 1;
se = 0)
Temporal (11) Visual field defect (4) Visual field defect (7)
Parietal (11) Behavioral anomalies (2)
Occipital (5)
Ataxia (7)
Vertigo and dizziness (2)
2110 Neurol Sci (2013) 34:2107–2116
123

Results
Peri-operative and post-operative outcomes
Early post-operative mortality
Three patients (2.9 %), all in Group 1, died in the first post-
operative week (Ibanez grade 4, Tables 4, 5). One patient
died because of uncontrolled cerebellar swelling after a
supracerebellar infratentorial approach for the removal of a
thalamic GBM. Post-op CT scan showed a complete
removal with a clean surgical cavity along with an
impressing cerebellar swelling that was probably due to
supracerebellar vein coagulation or thrombosis of the Galen
vein complex. Two patients died of pulmonary embolism
due to deep venous thrombosis (one female, obese patient,
Table 2 Patient demographics
in 104 patients: sex age, KPS,
presence of co-morbidities,
tumor diameter, MGMT (O(6)-
methylguanine-DNA
methyltransferase) status,
recurrence
Variable No. of patients
Group 1 Group 2 Total
Sex
Male 37 26 63
Female 21 20 41
Age
Men (mean) 61 62 61
Female (mean) 57 54 56
Older age ([72 years) 12 6 18
KPS \ 80 20 5 25
Comorbidities
Cardiopathy 12 8 20
Diabetes 4 6 10
Obesity 2 6 8
COPD 2 2 4
Hypertension 8 10 18
Tumor diameter
C4 cm 39 20 59
MGMT status
Presence of methylation 14 4 18
Absence of methylation 17 10 27
Recurrence 22 11 33
No. of patients operated two times by
the same operator (FP)
10 4 14
No. of patients operated on only for the
recurrence by the operator FP
10 9 19
Table 3 Post-operative
clinical result after surgery in
101 patients
Tumor resection and post-operative morbidity Group 1 (55 cases) Group 2 (46 cases)
Good: total removal with clinical and neurological
improvement or absence of new neurological
deficit (60.39 %)
29/55 (52.7 %) 32/46 (69.5 %)
GTR 29 (52.7 %) GTR 32 (69.5 %)
Acceptable: subtotal removal or presence of new
non-invalidating neurological deficit or new
invalidating neurological deficits that improved
within 30 days (31.68 %)
22/55 (40 %) 10/46 (21.7 %)
GTR 4 (7.3 %) GTR 2 (4.3 %)
STR 17 (30.9 %) STR 8 (17.3 %)
PR 1 (1.8 %) PR 0 (0 %)
Poor: partial removal or disability (7.92 %) 4/55 (7.3 %) 4/46 (8.7 %)
GTR 0 (0 %) GTR 4 (8.7 %)
STR 4 (7.3 %) STR 0 (0 %)
PR 0 (0 %) PR 0 (0 %)
Neurol Sci (2013) 34:2107–2116 2111
123

and one male, hemiplegic after surgery) despite adequate
antithrombotic therapy (seleparin 0.4 ml). The early post-
operative mortality in Group 1 did not result in a statistically
significant difference from the one in Group 2 (0 %)
(p = 0.25) because of limited sample sizes. All three of
these patients were excluded from further analysis.
Morbidity
The detail of complications is illustrated in Tables 4 and 5.
Four cases required a reoperation for the treatment of a
surgical complication (2 wound revisions for infection, 1
sub-dural and 1 intracerebral hematoma evacuation). No
Table 4 Complication grades: Ibanez model
Grades Surgical complications Medical complications
Group 1 Group 2 Group 1 Group 2
Ia (9) T new speech disorder (2) Subgaleal CSF collection (1)
P new speech disorder and
new motor deficits (2)
P visual field defect (1)
P visual field defect (1)
T New motor deficits (1)
P new motor deficit (1)
Ib (21) Seizures (4) Seizures (2) Steroid diabetes (3)
DVT (1)
Urinary tract infection (2)
arrhythmia (1) cardiac
ischemia (1)Fever (1)
CSWS (1)
Post-operative brain edema (1)
Wound infection (1)
Thalamic pain (1)
Middle cerebral artery
infarction (1)
CFS infection (1)
IIa (2) Subgaleal CSF accumulation
(lumbar puncture) (1)
Subgaleal CSF collection
(lumbar puncture) (1)
IIb (2) Wound infection (1) Wound infection (1)
IIIa (1) Subdural hematoma (1)
IIIb (4) Intracerebral hematoma (1) DVT and pulmonary
embolism (2)
lung distress (1)
IV (3) Cerebellar swelling (1) DVT and pulmonary
embolism (2)
T transient, P permanent, DVT deep vein thrombosis, CSF cerebrospinal fluid, CSWS cerebral salt wasting syndrome
Table 5 Neurosurgical complication following Landriel-Ibanez classification
Grade I Any non-life-threatening deviation from normal postoperative course, not requiring invasive treatment
Grade Ia Complication requiring no drug treatment
Grade Ib Complication requiring drug treatment
Grade II Complication requiring invasive treatment such as surgical, endoscopic, or endovascular interventions
Grade IIa Complication requiring intervention without general anesthesia
Grade IIb Complication requiring intervention with general anesthesia
Grade III Life-threatening complications requiring management in ICU
Grade IIIa Complication involving single organ failure
Grade IIIb Complication involving multiple organ failure
Grade IV Complication resulting in death
Surgical complications Adverse events that are directly related to surgery or surgical technique
Medical complications Adverse events that are not directly related to surgery or surgical technique
2112 Neurol Sci (2013) 34:2107–2116
123

statistically significant difference was found between
Groups 1 and 2 even though there was a definite trend
toward a higher percentage of new post-operative neuro-
logical deficits in Group 1. Age, sex and co-morbidities
also did not appear to have any effect on short-term sur-
gical outcomes in a statistically significant way. The short
term results of surgery on 101 patients could be graded as
‘good’ in 61 cases (60.4 %), ‘acceptable’ in 32 (31.6 %)
and ‘poor’ in 8 cases (7.9 %) (Table 3). There was no
statistically significant difference between Group 1 and
Group 2, even though there was a trend towards better post-
operative results in Group 2 (p = 0.14).
Survival
The mean overall survival time in 84 patients operated on the
first time by FP was 19.8 months (range 1.7–49.3 months;
median 17.7 months). Of these 84 patients, 16 were re-
operated on a second time for their recurrence (also by FP).
Overall survival rates at 6, 12, 18 and 24 months were 86.9,
65.4, 47.6 and 32 %, respectively. Patients in Group 2
showed a trend towards longer overall survival times (20.4
vs. 19.5 months) although this trend lacked any statistical
significance (p = 0.69) (Fig. 2).
In a univariate analysis after controlling for pre-opera-
tive factors known to be associated with survival, none of
the following parameters were found to be associated with
decreased survival: (1) presence/absence of motor deficits;
(2) presence/absence of language deficits (3) involvement
of thalamus/basal ganglia/brainstem; (4) involvement of
corpus callosum; (5) encasement of major brain arteries;
(6) KPS score \ or C80; (7) tumor size \ or C4 cm; (8)
MGMT status. The only parameter that seemed to be sig-
nificantly associated with increased survival time was the
extent of tumor excision. Patients with a total tumor
removal had a mean survival that was 9 months longer
than those with a partial excision (24 vs. 15.1 months,
p = 0.004). Patient age only showed statistical significance
at a threshold of 72 years (mean survival in patients
younger than 72: 21.2 months vs. 12.7 months in the
Group of patients older than 72, p = 0.03).
Recurrent GBMs
In this series 31 patients underwent surgery for a recurrent
tumor. Of these, 14 received their first surgery by the
author (FP) and 17 were selected for surgery when they
came from other hospitals. Of these, 19 were included in
Group 1 and 12 in Group 2. The mean overall survival time
was 13.6 months after the second surgery (Group 1:
11.8 months; Group 2: 17.5 months; p = 0.4).
The short-term results of surgery in these 31 patients
could be graded as ‘good’ in 12 cases (38.7 %); ‘accept-
able’ in 12 cases (38.7 %); and as ‘poor’ in 7 cases
(22.6 %). There was no statistically significant difference
between Group 1 and Group 2 (p [ 0.05).
Quality of life
Quality of life questionnaires were recorded in 93 out of
the 101 patients/families that were studied. In the Group 1,
in 55 % of cases the long-term results, in terms of quality
of life, were graded as either ‘excellent’ or ‘good’ (Fig. 3).
In Group 2 the percentage was higher (72 %), although it
lacked any statistical significance (p = 0.09) (Fig. 3).
Out of 93 patients, 89.2 % of these patients reported
positive experiences from their surgery. Only 10.8 %
reported that on the basis of their post-surgery status they
would not go through surgery again.
As far as recurrent cases are concerned, out of 31 patients,
most of them (74.2 %) found the second operation useful
and gave a positive opinion after that second surgery.
Fig. 2 Probability of survival in patients in Group 1 versus patients
in Group 2 in relation to the number of months after the first
operation. Rates were calculated with the Kaplan–Meier method. The
average survival in 84 patients was 19.5 (median 14.1 month) and
20.4 (median 19 months) months in Group 1 and in Group 2,
respectively (p = 0.69). Seventeen patients were excluded because
operated on the first time in another hospital. Out of these 84 patients
12 were re-operated a second time for their recurrence by the same
surgeon; 9 patients in Group 1 and 3 patients in Group 2
Neurol Sci (2013) 34:2107–2116 2113
123

Discussion
At present the surgical indications for high grade gliomas
are far from being uniform and are not based upon sound
evidence-based criteria [3, 12–15]. The significant pre-
operative prognostic factors that exist are: age (cut-off at
72 years in this series); Karnofsky score; multifocality and
tumor volume [14, 16–18].
Surgery is obviously one of the weapons that are available
to improve QoL and to slow the fatal evolution of the disease.
This is doubtlessly the reason why patients and their relatives
see surgery as a source of great hope. But aggressive surgery
remains debated and is not always offered in high risk glio-
mas because of number of reasons: poor prognosis, inability
to tolerate long surgical times, poor physiological and
neurophysiological compliance, increased risk of compli-
cations (venous thrombosis and arterial thrombosis of major
cerebral vessels) [14, 16, 17, 19].
Two of the criteria that are used by some surgeons to
define the ‘‘operability’’ of gliomas are the site of the tumor
and the degree of its extension [20–22]. Tumors that we
classified as complex in this series are often labeled as
‘inoperable’ because of the supposed much higher risk of
post-operative complications and invalidating neurological
deficits. The results of our retrospective analysis challenge
this idea and show that most of these patients benefit from a
gross total removal of the tumor (Fig. 1) with, in our
opinion, acceptable levels of mortality and morbidity.
Indeed, in our series, 68 % of the cases in Group 1 had a
post-operative course that revealed no new neurological
deficits. Although the difference between Group 1 and
Group 2 did not result to be statistically significant, the
number of patients with temporary neurological deficits in
the post-operative period was noticeably higher (18 vs. 8
pts) in Group 1 and appeared to be directly related to the
surgical manipulation of eloquent brain. Since the purpose
of surgery is to improve survival without affecting the level
of autonomy, what should be taken into account is not the
presence of temporary deficits but the absence of difference
between the two Groups in terms of permanent neurolog-
ical deficits (7.3 vs. 8.7 %) with loss of autonomy and need
for assistance.
In addition, it should be taken into account a specific
flaw of this study coming from the fact that the long-term
evaluation of the clinical results was influenced not only by
surgery, but also by the effects of radiotherapy, chemo-
therapy and possible disease progression.
As far as the difference in death rate (3 in Group 1 and 0
in Group 2) is concerned, we have to consider that only one
patient died because of the surgical procedure (post-oper-
ative cerebellar swelling and brainstem compression). The
other two patients died following a medical complication
(pulmonary embolism) that can occur following any neu-
rosurgical procedure for GBM removal, regardless of its
complexity.
The survival times of simple cases were found to be not
at all statistically significantly different from the survival
times observed in complex cases (Group 1:19.51 months
vs. Group 2: 20.4 months). This confirms the idea that
tumor removal has the potential to help patients wherever
the tumor is located. We also observed that a gross total
tumor removal was found to help patients regardless of
having an advanced age [18, 19, 23].
The current knowledge today is that among all patients
with GBM, those with unresectable tumors are considered
to have the worst prognosis. The treatment strategy for
these patients is, however, poorly documented in the lit-
erature, in spite of the fact that this subgroup of patients
can represent up to 35–40 % of all GBM patients. The need
to provide optimal treatment to these patients is evident.
We know that surgical techniques have significantly
improved in the last two decades. Kelly observed in 2004
that even though the survival of GBM patients is not rad-
ically altered by surgery, today’s surgical methods are far
less likely to ‘hurt the patient’ [24]. These circumstances
provide evidence that a better definition of surgical results
and overall survival prognostic factors is needed to refine
surgical indication rather than considering tumors simply
Fig. 3 Data of long-term
results obtained in 93 patients/
relatives after phone interview.
a Group 1; b Group 2
2114 Neurol Sci (2013) 34:2107–2116
123

operable or inoperable. Our results would appear to be
completely aligned with Kelly’s opinion. While it is clear
that surgery cannot ‘cure’ glioblastoma because of that
tumor’s intrinsic biological features, a complete removal
does seem to be associated with a longer disease-free
period and enhanced survival [4, 23, 25, 26]. In our view,
therefore, these complex glioblastoma cases are worthy of
every technical effort that promises to remove as much of
the tumor as possible without inflicting any new neuro-
logical deficits that could influence the QoL. There are now
many new strategies that can make this surgical possibility
a reality. These include: intraoperative image guidance;
inside-out tumor removal fluorescence guided surgery; ICG
videoangiography with special attention to the microsur-
gical respect of ‘passing’ arterial vessels; the use of new
hemostatic agents; neurophysiological mapping; and awake
surgery [25, 27–29]. All of these advances offer significant
improvements in helping the surgeon to maximize resec-
tion while minimizing the risks of damage to the healthy
residual brain. And in our estimation they all conspire to
invite surgeons to push the limits of neurosurgery beyond
the boundary conditions that, in the past, were used to
define tumors as ‘inoperable’ [15]. Even if we did not find
statistical significance, is noticeable that the patients in
Group 2 present a better result in terms of QoL and a higher
percentage (55 % in Group 1 vs. 72 % in Group 2) of
patients was able to work full time at the follow-up (grade
excellent and good according to Heros classification), thus
proving that also radio- and chemotherapy influence the
QoL.
Though a larger number of observations would obvi-
ously be much more desirable our findings do seem to be
making an obvious point. Supporting that point is the fact
89.2 % of our patients and their relatives gave a positive
opinion regarding surgery. These opinions, along with the
fact that the results of the entire series compare well with
the results that are currently found in the literature, provide
a rationale to encourage us to continue this investigation of
the legitimate and valid limits of modern surgery [30, 31].
In addition, we believe that these results, in spite of the
intrinsic limits of this analysis (retrospective study, limited
sample with small subgroups, single surgeon in a single
institution, lack of a control group), support the idea that
a patient with a ‘complex tumor’ should be evaluated
in high-volume modern oncologic centers before being
labeled as ‘inoperable’.
Conclusions
On the basis of our findings, we can argue that some
clinical and radiological aspects ‘per se’ do not constitute a
valid or useful reason to label a patient as harboring an
‘inoperable’ tumor. Further prospective studies that are
specifically aimed at a systematic stratification of the risks
of GBM surgery are needed, and hopefully, will be
conducted.
Acknowledgments We thank heartily Dr. Allen Fertziger (Adjunct
Professor, Honors Department, University of Maryland) for assistance
in revising the manuscript.
Conflict of interest We have disclosed potential conflicts of
interest.
References
1. Stummer W, Meinel T, Ewelt C et al (2012) Prospective cohort
study of radiotherapy with concomitant and adjuvant temozolo-
mide chemotherapy for glioblastoma patients with no or minimal
residual enhancing tumor load after surgery. J Neuro oncol (Epub
ahead of print)
2. Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate
analysis of 416 patients with glioblastoma multiforme: prognosis,
extent of resection, and survival. J Neurosurg 95(2):190–198
3. Stummer W, van den Bent MJ, Westphal M (2011) Cytoreductive
surgery of glioblastoma as the key to successful adjuvant thera-
pies: new arguments in an old discussion. Acta Neurochir (Wien)
153(6):1211–1218 (Epub 2011 Apr 9)
4. McGirt MJ, Chaichana KL, Gathinji M et al (2009) Independent
association of extent of resection with survival in patients with
malignant brain astrocytoma. J Neurosurg 110(1):156–162
5. Cote R, Battista RN, Wolfson C et al (1989) The Canadian
Neurological Scale: validation and reliability assessment. Neu-
rology 39:638–643
6. Brott T, Adams HP Jr, Olinger CP et al (1989) Measurements of
acute cerebral infarction: a clinical examination scale. Stroke
20(7):864–870
7. Bizzi A (2009) Presurgical mapping of verbal language in brain
tumors with functional MR imaging and MR tractography.
Neuroimaging Clin N Am 19(4):573–596
8. Stupp R, Mason WP, Van den Bent MJ et al (2005) Radiotherapy
plus concomitant and adjuvant temozolomide for glioblastoma.
N Engl J Med 352:987–996
9. Berger MS (1994) Lesions in functional (‘‘eloquent’’) cortex and
subcortical white matter. Clin Neurosurg 41:444–463
10. Landriel Ibanez FA, Hem S, Ajler P et al (2011) A new classi-
fication of complications in neurosurgery. World Neurosurg
75(5):709–715 (discussion 604–11)
11. Heros RC, Korosue K, Diebold PM (1990) Surgical excision of
cerebral arteriovenous malformations: late results. Neurosurgery
26:570–578
12. Ashby LS, Ryken TC (2006) Management of malignant glioma:
steady progress with multimodal approaches. Neurosurg Focus
20:E3
13. Devaux BC, O’Fallon JR, Kelly PJ (1993) Resection, biopsy, and
survival in malignant glial neoplasms. A retrospective study of
clinical parameters, therapy, and outcome. J Neurosurg 78:767–
775
14. Vuorinen V, Hinkka S, Farkkila M et al (2003) Debulking or
biopsy of malignant glioma in elderly people—a randomised
study. Acta Neurochir (Wien) 145:5–10
15. Ferroli P, Acerbi F, Franzini A (2011) The dawn of the hodotopic
era in neurosurgery: is there a need to upgrade the operability
criteria for brain tumors? World Neurosurgery 75(5–6):571–572
16. Chaichana K, Parker S, Olivi A et al (2010) A proposed classi-
fication system that projects outcomes based on preoperative
Neurol Sci (2013) 34:2107–2116 2115
123

variables for adult patients with glioblastoma multiforme. J Neu-
rosurg 112(5):997–1004
17. Burger PC, Green SB (1987) Patient age, histologic features, and
length of survival in patients with glioblastoma multiforme.
Cancer 59(9):1617–1625
18. Ewelt C, Goeppert M, Rapp M et al (2011) Glioblastoma mul-
tiforme of the elderly: the prognostic effect of resection on sur-
vival. J Neurooncol 103(3):611–618 (Epub 2010 Oct 16)
19. Gulati S, Jakola AS, Johannesen TB, Solheim O (2012) Survival
and treatment patterns of glioblastoma in the elderly: a popula-
tion-based study. World Neurosurg 78(5):518–526
20. Teo C, Broggi M (2010) Surgical outcome of patients considered
to have ‘‘inoperable’’ tumors by specialized pediatric neuro-
oncological multidisciplinary teams. Childs Nerv Syst 26(9):
1219–1225 (Epub 2010 Jun 19)
21. Lange OF, Haase KD, Scheef W (1987) Simultaneous radio- and
chemotherapy of inoperable brain tumours. Radiother Oncol
8(4):309–314
22. Fazeny-Dorner B, Wenzel C, Veitl M et al (2003) Survival and
prognostic factors of patients with unresectable glioblastoma
multiforme. Anticancer Drugs 14(4):305–312
23. Brandes AA, Compostella A, Blatt V et al (2006) Glioblastoma in
the elderly: current and future trends. Crit Rev Oncol Hematol
60(3):256–266 (Epub 2006 Oct 5)
24. Kelly PJ (2004) Technology in the resection of gliomas and the
definition of madness. J Neurosurg 101(2):284–286
25. Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence
guided surgery with 5-aminolevulinic acid for resection of
malignant glioma: a randomised controlled multicentre phase III
trial. Lancet Oncol 7:392–401
26. Sanai N, Polley MY, Berger MS et al (2011) An extent of
resection threshold for newly diagnosed glioblastomas. Neuro-
surg 115(1):3–8 (Epub 2011 Mar 18)
27. Ferroli P, Tringali G, Acerbi F et al (2010) Brain surgery in a
stereoscopic virtual reality environment: a single institution’s
experience with 100 cases. Neurosurgery 67(3 Suppl Operative):
ons79–84 (discussion ons84)
28. Ferroli P, Acerbi F, Albanese E et al (2011) Application of
intraoperative indocyanine green angiography for CNS tumors:
results on the first 100 cases. Acta Neurochir Suppl 109:251–257
29. Leclercq D, Duffau H, Delmaire C et al (2010) Comparison of
diffusion tensor imaging tractography of language tracts and intra-
operative subcortical stimulations. J Neurosurg 112(3):503–511
30. Olson JJ, Fadul CE, Brat DJ et al (2009) Management of newly
diagnosed glioblastoma: guidelines development, value and
application. J Neurooncol 93(1):1–23 (Epub 2009 May 9)
31. Olson JJ, Ryken T (2008) Guidelines for the treatment of newly
diagnosed glioblastoma: introduction. J Neurooncol 89(3):255–
258 (Epub 2008 May 23)
2116 Neurol Sci (2013) 34:2107–2116
123