Maria Antonietta Gambacorta - Congressi · PDF fileAttualità nei trattamenti integrati...
Transcript of Maria Antonietta Gambacorta - Congressi · PDF fileAttualità nei trattamenti integrati...

AttualitaneitrattamentiintegratidelcarcinomadelRettolocalmenteavanzato:versolapreservazioned’organoIlpuntodivistadelradioterapistaoncologo
MariaAntoniettaGambacorta
APPROPRIATEZZADELL’IMAGING
NEITUMORIDELRETTO

pCR andOrgan Preservation
• Themeaning ofCR
• Howtoachieve CR• What toirradiate• Howtopredict

pCR after CRT
Protocol pCR Concomitant CT CumulativepCR
EORTC22921 13.7*
5FUFFCD9203 11.4
STAR 16
5FU
and
oxaliplatin
≈15%ACCORD 19.2
NASBP R04 20.9
CAO-ARO-AIO 04 16.5
*pT0

LC-CRT
The meaning of CR after CRT

pCR andlongterm outcomes:RCT
Articles
986 www.thelancet.com/oncology Vol 16 August 2015
445 patients who actually received adjuvant fl uorouracil and leucovorin and oxaliplatin, as compared with 100 (21%) of 470 patients who actually received adjuvant fl uorouracil alone. Grade 3 or worse diarrhoea was the most frequent chronic adverse eff ect in both groups (31 [7%] in the investigational group vs 42 [9%] in the
control group). The incidence of grade 3–4 sensory neuropathy in the fl uorouracil and leucovorin and oxaliplatin group decreased from 41 (10%) patients during treatment to 13 patients (3%) at 1 year follow-up. Among the 326 patients in the fl uorouracil and leucovorin and oxaliplatin group having sphincter-sparing surgery,
Figure 4: Disease-free survival in the intention-to-treat population by patient subgroups according to pretreatment and surgical or pathological factors after preoperative chemoradiotherapyThe size of the quadrats represents the proportion of patients. HR=hazard ratio. ECOG=Eastern Cooperative Oncology Group.
52/23058/23849/145
112/43447/179
115/48343/123
61/249
83/302
14/55
145/57114/41
33/146123/452
12/1149/39
29/16092/260
9/17
75/416
45/133
30/42
135/567
6/15
9/10419/14840/15457/154
98/398
5/3145/151
159/613
84/24164/23350/149
149/44049/183
136/47558/141
87/216
84/336
24/64
169/56927/50
58/159134/451
6/837/39
41/183120/27817/26
94/423
53/131
44/60
167/584
7/9
6/8130/17648/14871/169
105/416
10/3067/152
198/623
HR (95% CI)
0·61 (0·43–0·86)0·87 (0·61–1·24)1·06 (0·71–1·58)
0·73 (0·57–0·93)0·98 (0·65–1·46)
0·80 (0·62–1·02)0·86 (0·58–1·28)
0·57 (0·41–0·79)
1·10 (0·81–1·49)
0·64 (0·33–1·25)
0·83 (0·67–1·04)0·62 (0·32–1·18)
0·56 (0·36–0·86)0·91 (0·71–1·16)
1·52 (0·57–4·05)
1·40 (0·52–3·76)
0·77 (0·48–1·24)
0·78 (0·60–1·03)
0·76 (0·34–1·70)
0·78 (0·58–1·06)
0·82 (0·55–1·22)
1·09 (0·65–1·81)
0·80 (0·64–1·01)
0·40 (0·13–1·25)
1·20 (0·43–3·36)0·72 (0·40–1·28)0·74 (0·49–1·13)0·89 (0·63–1·28)
0·98 (0·74–1·29)
0·46 (0·16–1·35)0·58 (0·39–0·85)
0·79 (0·64–0·98)
0·2 0·5 1·0 2·0 3·0 4·0Hazard ratio
Age (years)<6161–70>70
SexMaleFemale
ECOG performance status01–2
Location from anal verge0–5 cm>5–10 cm>10 cm
cT categorycT2–3cT4
cN categorycN0cN+
ypT categoryypT0ypTis/T1ypT2ypT3ypT4
ypN categoryypN0ypN1ypN2
Completeness of local tumour resectionR0R1
TNM stageypT0N0Stage IStage IIStage III
Type of surgeryLow anterior resectionIntersphincteric resectionAbdominoperineal resection
TotalAll
Favours investigational group Favours control group
Investigational group(events/n)
Control group(events/n)
Rodel C et al. Lancet Oncol 2015

pCR andlongterm outcomes:
pooled analyses
Maas M et al. Lancet Oncol 2010

cT3orcT2low
Long Course RT-CT
Surgery descalation
Pucciarellietal,S.etalDis ColonRectum 2013GérardJPetal.JClin Oncol 2014Vuong Tetal.Semin ColonRectal Surg 2010Maas MetalJClin oncol 2011Appelt A.LancetOncol 2015
cCR

Sexual function (male*)
Stephens etalJCO2010
surgery
RT
*Only 11%ofwomen completed thequestionnaire at 2yrs

Sphincter function
Unintentional releaseofstool
Stephens etalJCO2010

pCR andOrgan Preservation
• Themeaning ofCR• Howtoachieve CR• What toirradiate• Howtopredict

Howtoachieve CR
• Chemotherapy intensification
• Radiotherapy intensification
• TIMECRT Surgery

CTintensification
During RT
‘New’CTagents
Targettherapies
Sequence oftherapies
TotalNeoadjuvant Therapy

CTintensification during RT:
‘Newagents’

BytheCourtesyofC.Rodel – ESTROCourse2016
Targettherapies

By the Courtesy of C.Rodel – ESTRO Course 2016
Targettherapies

CTintensification:
TotalNeoadjuvant Treatment
Fernandez-Martoz Cetal.JCO2010
Conclusion: Both treatment approaches yield similar outcomes. Given the lower acute toxicity and improved compli-ance with induction CT compared with adjuvant CT, integrating effective systemic therapy before CRT and surgery is apromising strategy and should be examined in phase III trials.Key words: locally advanced rectal cancer, induction chemotherapy, phase II randomized trial, adjuvant chemotherapy
introductionThe role of adjuvant chemotherapy (CT) after preoperative che-moradiation (CRT) has not been well established in publishedrandomized trials [1–4]. The low compliance with CT followingpreoperative CRT and surgery as well as the lack of a reliablemethod to select patients, might contribute to these negativeresults. Furthermore, the British Chronicle and the DutchSCRIPT-PROCTOR trials were terminated early because pooraccrual and consequent insufficient statistical power.More recently, the Korean ADORE multicenter, randomized,
phase II study included poor prognostic rectal cancer patientswho received preoperative CRT and had pathological stage II(ypT3-4N0) or III (ypTanyN1-2) disease. Three-year disease-free survival (DFS) was significantly improved in patientstreated with adjuvant FOLFOX compared with those receivingadjuvant 5-fluorouracil (5-FU) and leucovorin (71.6% versus62.9%, P = 0.047). Interestingly, 96% of patients completed theplanned number of adjuvant CT cycles [5].
An alternative approach to improving outcome is to deliver in-duction CT before preoperative CRT. Induction CT may be asso-ciated with better treatment compliance and may allow fullsystemic doses of CT to be delivered. Our group completed a ran-domized phase II trial (GCR-3) comparing this approach withconventional preoperative CRT followed by surgery and post-operative adjuvant CT. Primary results were reported in 2010.Compared with postoperative adjuvant capecitabine oxaliplatin(CAPOX), induction CAPOX before CRT had similar pathologic-al complete response (pCR) and complete resection rates while atthe same time achieving more favorable compliance and toxicityprofiles [6]. Given these advantages, this induction strategy isnow considered a new treatment alternative for patients withhigh-risk rectal cancer in some European institutions and theUnited States [7]. We report long-term outcomes including localrecurrence, distant recurrence and survival results after a medianfollow-up of 69 months.
108 patients randomized
49 (94%) patients commenced CRT
3 ineligible patients excluded
46 (89%) patients underwent surgery
45 R0 resections
1 R1 resection
37 (71%) patients commenced adjuvant CT
9 did not receive any cycle
7 received 1-2 cycles
2 received 3 cycles
28 received 4 cycles
54 (96%) patients commenced CRT
54 (96%) patients commenced induction CT
1 received 1 cycle
1 received 3 cycles
52 received 4 cycles
54 (96%) patients underwent surgery
48 R0 resections
2 R1 resections
2 R 2 resections
2 ineligible patients excluded
Follow-up07/2013
Life Status Know n = 55Tumor status know = 55
Follow-up07/2013
Life Status Know n = 50Tumor status know = 50
Arm B: Induction CT56 pts 100%
CT CTRT Surgery
Arm A: Adjuvant CT52 pts 100%
CTRT Surgery CT
Figure 1. Consort diagram. CRT, chemoradiation; CT, chemotherapy.
Volume 26 | No. 8 | August 2015 doi:10.1093/annonc/mdv223 |
Annals of Oncology original articles
by guest on Decem
ber 10, 2016http://annonc.oxfordjournals.org/
Dow
nloaded from

CT)—are listed in Table 1. The predominant clinical disease stage wascT3N!. After random assignment, arm B included more patientswith T4 lesions than arm A (13% v 6%) and also more grade 3 tumors(11% v 2%). Arm A included more patients with a threatened circum-ferential resection margin than arm B (10% v 0%). Otherwise, patientcharacteristics were well balanced between the two arms.
The number of eligible patients who commenced treatment was49 (94%) of 52 in arm A and 54 (96%) of 56 in arm B. Overall, 46patients (89%) in arm A and 54 (96%) in arm B underwent surgery.When patients who had the appropriate dose reductions were in-cluded, more patients in arm B than arm A completed the study as perprotocol (91% v 54%; P " .0001). A total of nine patients (17%) inarm A and 1 patient (2%) in arm B discontinued study treatmentbecause of adverse events (P # .006). The percentages of patients whodiscontinued treatment for other reasons were similar in the twogroups (Table 2).
Efficacy ParametersA total of 46 patients in arm A, after preoperative CRT, and 54
patients in arm B, after induction CT and preoperative CRT, under-went surgery. On the basis of an intent-to-treat analysis, a pCR(ypT0N0M0) was achieved in seven patients in arm A (13%; CI 95%,5.6% to 25.8%) and in eight patients in arm B (14%; CI 95%, 6.4% to
26.2%). Downstaging (defined as lower pathologic T stage comparedwith the pretreatment clinical T stage) was observed in 30 patients(58%) in arm A and in 24 patients (43%) in arm B. An R0 resectionwas achieved in 45 patients (87%) in arm A and in 48 patients (86%) inarm B. In arm A, the tumor regression grade (TRG) of the primarytumor (ypT0) was TRG 4 in seven patients, and an additional 22patients showed tumor regression of greater than 50% of the tumormass (TRG 3). In contrast, in arm B, eight patients had a TRG of 4, and20 patients had a TRG of 3 (Table 3).
Table 1. Baseline Demographic and Clinical Characteristics for theTotal Patient Group
Characteristic
Arm A:Post-
operativeAdjuvant
CT(n # 52)
Arm B:Induction
CT(n # 56)
No. % No. %
Age, yearsMedian 62 60Range 42-75 38-76
SexMale 34 65 39 70Female 18 35 17 30
ECOG status0 36 69 33 591 15 29 22 392 — 1 2Unknown 1 2 —
Locally advanced rectal cancer definitionby MRI $ US
cT4 resectable 3 6 7 13cT3 lower third (! 6 cm from anal
verge) tumors 12 23 18 32CRM threatened or involved,
mid-rectal cancer 5 10 —Any cT3N! 31 59 31 55Missing 1 2 —
Pathologic gradeNot otherwise specified 12 23 11 201: well differentiated 12 23 11 202: moderately differentiated 27 52 28 503: poorly differentiated 1 2 6 11
Abbreviations: CT, chemotherapy; ECOG, Eastern Cooperative OncologyGroup; MRI, magnetic resonance imagine; US, ultrasound; CRM, circumfer-ential resection margin.
Table 2. Treatment Received and Reasons for Discontinuation
Variable
Arm A:Post-
operativeAdjuvant
CT(n # 52)
Arm B:Induction
CT(n # 56)
PNo. % No. %
Completion of study treatment perprotocol 28 54 51 91 " .0001
Discontinuation of study treatment! 22 44 5 9 " .001Reason for discontinuation
Progression of disease 1 2 0 .93Adverse event 9 17 1 2 .006Investigator decision/unfit for CT 4 6 0 .05Death 3 6 3 6 1Toxicity 2 4 2 4Other† 1 2 1 2Consent withdrawn 5 10 1 2 .1
Abbreviation: CT, chemotherapy.!Two patients in arm A did not have data available.†One patient in arm A committed suicide; one patient in arm B had
major depression.
Table 3. End Points for the Total Patient Group
End Point
Arm A:Post-
operativeAdjuvant
CT(n # 52)
Arm B:Induction
CT(n # 56)
P !No. % No. %
pCR 7 13 8 14 .9495% CI, % 5.6 to 25.8 6.4 to 26.2
Downstaging 30 58 24 43 .1395% CI, % 43.2 to 71.3 29.7 to 56.8
R0 resection rates 45 87 48 86 .40TRG†
4: complete regression 7 15 8 15 .883: % 50% of tumor mass 22 48 20 372: " 25%-50% of tumor mass 11 24 13 241: " 25% of tumor mass 2 4 3 60: no regression 1 2 3 6Not otherwise specified 3 7 7 13
Abbreviations: CT, chemotherapy; pCR, pathologic complete response; TRG,tumor regression grade.
!Fisher’s exact test.†The denominators were the patients who actually underwent resection.
Fernandez-Martos et al
862 © 2010 by American Society of Clinical Oncology JOURNAL OF CLINICAL ONCOLOGY
Downloaded from ascopubs.org by 185.11.153.252 on December 10, 2016 from 185.011.153.252Copyright © 2016 American Society of Clinical Oncology. All rights reserved.
Preoperative treatmentintensification:
TotalNeoadjuvant Treatment
Fernandez-Martoz Cetal.JCO2010

Howtoachieve CR
• Chemotherapy intensification
• Radiotherapy intensification
• TIMECRT Surgery

RTintensification

D50,TRG1= 92.0Gy
D50,TRG1-2= 72.1Gy
Appelt ALetal.Int.JRadiat Oncol Biol Phys 2013
Reliability of RT for cure

Burbach etal.Radiother Oncol 2014
RT intensification: dose> 60Gy
Fig. 2. Meta-analysis forest plot of pCR-rates and pooled estimate in comparison to a reference line of control group (14.8%) [3] (pCR = pathological complete response).
6 Impact of radiotherapy boost on pathological complete response in rectal cancer
pCR 20%
since in some patients surgery was omitted for other reasons suchas a worsened condition, newly diagnosed metastasis or patient’srefusal. Fourth, the pathologic assessment was different betweenstudies, and therefore prone to bias. Ten of 14 included studiesstandardized assessment, of which 3 explicitly used Mandard’sscore [35]. Others only mentioned that one pathologist assessedif there was ‘absence of viable tumor cells’ in the specimen. Fifth,destruction of solitary tumor cells may continue long after termi-nation of radiotherapy, indicating that timing of surgery impactsresponse assessment. Three studies have shown increased pCR-rates when surgery was postponed from 8 to 11 weeks post-radia-tion (from 11.5 to 14.0%) [61], and when shorter surgical intervalsare compared to intervals of >6–8, or >7, weeks (from 13.7 to19.5%, and 16 to 28.0% respectively) [62,63]. A relative risk of1.42 (1.19–1.68) for pCR was reported for intervals longer than6–8 weeks as compared to intervals shorter than 6–8 weeks. Nev-ertheless, in our data we did not see an association between inter-val-length and pCR-rate, presumably because pCR-rate variedlargely at each interval length with only a few studies available
per interval-length point in the analysis. Such variation is common,and therefore often observed in systematic reviews on pCR-ratesfollowing CRT [1,3,64]. To further investigate the impact of pro-longed intervals on pCR-rate and sphincter preservation, severalrandomized clinical trials are currently recruiting (GRECCAR6/NCT01648894 [65] and NCT01037049). Nonetheless, if such pre-sumed time-effects allow extrapolation to when doses are esca-lated, pCR-rates and organ-preservation might even furtherbenefit when longer intervals prove to be safe. Sixth, only a singlestudy reported interval between radiotherapy and brachytherapy,which did not allow further meta-analysis. Finally, acceleratedtreatment (higher dose per fraction, i.e. simultaneous integratedboost) increases the biological effective dose which may benefitresponse [66,67], especially when tumor-regrowth time is short[68,69]. Nevertheless, some of these accelerated schedules remainchallenging because of considerable toxicity [19,24,70–72] andperi/post-operative complications [72,73]. It is likely that such tox-icity originates from irradiation of surrounding tissues instead ofthe tumor, as a result of a previously acquired treatment plan
Fig. 3. Forest plot of available acute grade P3 toxicity and resectability with pooled estimate.
J.P.M. Burbach et al. / Radiotherapy and Oncology 113 (2014) 1–9 7
G3-4TOX10%

RTintensification
Valentini V et al. ESTRO-33-2014

RTintensification
Valentini V et al. ESTRO-33-2014

RTintensification
Modified from Valentini V et al. ESTRO-33-2014
pCR 24.4 23.8 ns

RTintensification
Valentini V et al. ESTRO-33-2014

Howtoachieve CR
• Chemotherapy intensification
• Radiotherapy intensification
• TIMECRT Surgery

Articles
www.thelancet.com/oncology Vol 16 August 2015 959
ProceduresThe trial consisted of a series of four sequential phase 2 study groups. In group 1, patients were treated with fl uorouracil-based chemoradiation and total mesorectal excision to establish the proportion of patients achieving a pathological complete response at baseline. In groups 2–4, patients received two, four, or six cycles of mFOLFOX6 between chemoradiation and total meso-rectal excision (fi gure 1). Patients in all study groups were treated with fl uorouracil 225 mg/m² per day by continuous infusion, 7 days per week throughout radiation. Fluorouracil infusion was given for 5−6 weeks, depending on the number of radiation boosts given. Radiation treatment was given once a day at 1·8 Gy per day, 5 days per week for 5 weeks, for a total of 45 Gy in 25 fractions, followed by a minimum boost of 5·4 Gy. In patients in whom the entire small bowel could be excluded from the fi nal cone down, a second boost of 3·6 Gy (54 Gy total cumulative dose) was given. A linear accelerator using a minimum 6 MV energy in three to four fi elds was delivered. Intensity-modulated radiation treatment was permitted if approved by the supervising radiation oncologist.
Patients in group 1 had surgery 6–8 weeks after chemo-radiation. Patients in groups 2–4 received cycles of mFOLFOX6 4−5 weeks after the completion of chemoradiation: patients in group 2 received two cycles, those in group 3 four cycles, and those in group 4 six cycles of mFOLFOX6. Each cycle consisted of racemic leucovorin 200 mg/m² or 400 mg/m², according to the discretion of the treating investigator, oxaliplatin
85 mg/m² in a 2-h infusion, bolus fl uorouracil 400 mg/m² on day 1, and a 46-h infusion of fl uorouracil 2400 mg/m². Patients had surgery 3–5 weeks after the last cycle of mFOLFOX6. To ensure patients in groups 2–4 were not placed at risk of disease progression during the lengthened chemoradiation-to-surgery interval, tumour response was assessed by Response Evaluation Criteria in Solid Tumors guidelines16 during the neoadjuvant treatment course. Patients with progressive or stable disease at interim assessment did not receive additional mFOLFOX6 and had total mesorectal excision without delay.
Surgery was done according to the principles of sharp mesorectal excision. Specimens were assessed according to the recommendations of the Association of Directors of Anatomic and Surgical Pathology.17 Postoperative chemotherapy to complete a total of eight cycles of mFOLFOX6 was recommended, but not dictated by the trial, and was delivered at the discretion of the treating physician.
OutcomesThe primary endpoint of the study was the proportion of patients achieving a pathological complete response (defi ned as the absence of tumour cells in the surgical specimen, both at the primary tumour site and at regional lymph nodes) in each study group. We also collected information on the proportion of patients who achieved pathological partial response (defi ned as having at least a 30% decrease in tumour width, or length in circumferential tumours), stable disease (between a 30% decrease and a 20% increase), and progressive disease (at
Figure 1: Trial protocolRadiotherapy was given 5 days per week for 5 weeks (arrows) for a total of 45 Gy with a minimum boost of 5·4 Gy. Fluorouracil was given as a 225 mg/m² per day continuous infusion for 7 days per week during radiation therapy for 5−6 weeks, depending on the number of radiation boosts given. mFOLFOX6 was given in 2-week cycles of leucovorin 200 mg/m² or 400 mg/m² and oxaliplatin 85 mg/m² in a 2-h infusion, bolus fl uorouracil 400 mg/m² on day 1, and a 46-h infusion of fl uorouracil 2400 mg/m². *Interim assessments were done by proctoscopic examination; total mesorectal excision was done if the patient had stable or progressive disease.
Group 1
Group 2
Group 3
Group 4 Total mesorectal excision
Total mesorectal excision
Total mesorectal excision
Total mesorectal excisionContinuous infusion fluorouracil + radiotherapy
Continuous infusion fluorouracil + radiotherapy
Continuous infusion fluorouracil + radiotherapy
Continuous infusion fluorouracil + radiotherapy
Rest
Rest
Rest
Rest
Rest
Rest
Rest
mFOLFOX6 (four cycles)
mFOLFOX6 (two cycles)
mFOLFOX6 (six cycles)
0 2 4 6 8 10 12 14 16 18 20 22 24 26Weeks
*
*
*
Preoperative treatmentintensification:
TotalNeoadjuvant Treatment
Garcia-Aguilar J etal.LancetOncol 2015
Primary end-point:ypCR
RCà 12cmfromAVcT3-4,N0;cT,N1-2

Preoperative treatmentintensification:
TotalNeoadjuvant Treatment
Garcia-Aguilar J etal.LancetOncol 2015
RCà 12cmfromAVcT3-4,N0;cT,N1-2
259enrolled patients
Articles
962 www.thelancet.com/oncology Vol 16 August 2015
during chemoradiation did not diff er between study groups (data not shown).
The proportion of patients experiencing adverse events during mFOLFOX6 treatment increased from group 2 to group 4. In group 2, two (3%) of 67 patients had grade 3 adverse events and one (1%) had a grade 4 adverse event; in group 3, 12 (18%) of 67 patients had grade 3 adverse events; in group 4, 18 (28%) of 65 patients had grade 3 adverse events and fi ve (8%) had grade 4 adverse events. The most common grade 3 or higher adverse events from mFOLFOX6 across study groups 2−4 were neutropenia in 11 patients (6%; fi ve in group 3 and six in group 4) and lymphopenia in seven patients (4%; three in group 3 and four in group 4). 18 (9%) of patients experienced neuropathy during mFOLFOX6 treatment, all of which were either grade 1 or 2. One patient had grade 1 neuropathy in group 2, six had grade 1 and one had grade 2 neuropathy in group 3, and nine had grade 1 and one had grade 2 neuropathy in group 4.
Table 2 summarises surgical results. The proportion of patients who received a sphincter-saving surgery and resection with negative margins was not signifi cantly diff erent between study groups (p=0·68 and p=0·089, respectively). The number of nodes examined and estimated blood loss were similar across all study groups (p=0·20 and p=0·62, respectively). Pelvic fi brosis, as measured by surgeon scoring from 1 (none) to 10 (maximum), increased in groups 2–4 (p=0·0001). However, the technical diffi culty of the operation, as
scored by the surgeon, was not signifi cantly diff erent across study groups (p=0·80).
No patient died during or after surgery in any study group. There was no signifi cant diff erence in the number of grade 3 or worse complications across study groups (all p>0·1; table 4). Grade 3 or worse complications were noted for nine (15%) patients in group 1, four (6%) patients in group 2, three (4%) patients in group 3, and six (9%) patients in group 4, when counting the maximum Clavien-Dindo grade complication for each patient. Of the 25 grade 3 or worse complications reported across all study groups, the most common were pelvic abscesses (seven patients: three in group 1, two in group 3, and two in group 4) and anastomotic leaks (seven patients: three in group 1, one in group 2, one in group 3, and two in group 4).
Using univariable logistic regression, we assessed known clinically relevant variables and study groups and their association with pathological complete response. The comparison of group 4 (the most intense regimen) with group 1 (the standard neoadjuvant regimen) showed a signifi cant association with pathological complete response (p=0·028; table 5). We then used multivariable logistic regression to model the probability of pathological complete response, examining whether a treatment eff ect on pathological complete response was present after adjusting for other known clinically relevant variables. In an intention-to-treat analysis that included study group, radiation dose, tumour stage, size, and distance to anal verge as variables, we found study group to be the only signifi cant predictor of pathological complete response (p=0·048; table 5). Patients in group 4 were signifi cantly more likely to achieve a pathological complete response than were patients in group 1 (odds ratio 3·49, 95% CI 1·39–8·75; p=0·011). In preplanned analyses, we tested two additional models in which we substituted study group for the treatment delivered as measured by cycles of mFOLFOX6 or the chemo radiation-to-surgery interval; both were signifi cant predictors of pathological complete response (p=0·028 and p=0·018, respectively).
Group 1 (n=60) Group 2 (n=67) Group 3 (n=67) Group 4 (n=65) p value
Time from start of chemoradiation to surgery (weeks) 14·2 (4·3) 17·1 (2·9) 21·0 (2·7) 25·2 (4·0) 0·0001
Time from end of chemoradiation to surgery (weeks) 8·5 (4·2) 11·1 (2·9) 15·4 (2·6) 19·3 (4·2) 0·0001
Sphincter-saving surgery 46 (77%) 50 (75%) 50 (75%) 44 (68%) 0·68
Ileostomy 38/46 (83%) 43/50 (86%) 47/50 (94%) 38/43 (88%)* 0·33
Resection with negative margins 59 (98%) 67 (100%) 64 (96%) 64 (100%)† 0·089
Number of nodes examined 12 (2–31) 14 (2–30) 13 (2–30) 11 (1–47) 0·20
Pelvic fi brosis‡ 2·4 (1·7) 3·9 (2·6) 4·4 (2·4) 3·9 (2·4) 0·0001
Technical diffi culty§ 4·6 (2·7) 4·9 (2·8) 5·1 (2·5) 4·8 (2·4) 0·80
Estimated blood loss (mL) 200 (50–1200) 225 (25–1500) 200 (50–1000) 150 (0–1000) 0·62
Data are mean (SD), number (%), n/N (%), or median (range). p values test the null hypothesis of equal means or proportions across study groups. *Information on whether an ileostomy was created or not was not available for one patient. †Data missing for one patient. ‡Scale ranges from 1 (none) to 10 (maximum). §Scale ranges from 1 (easy) to 10 (diffi cult).
Table 2: Surgical results
Group 1 (n=60)
Group 2 (n=67)
Group 3 (n=67)
Group 4 (n=65)
p value
Pathological complete response 11 (18%) 17 (25%) 20 (30%) 25 (38%) 0·0036
Partial response 44 (73%) 50 (75%) 46 (69%) 39 (60%) ··
Stable disease 5 (8%) 0 1 (1%) 1 (2%) ··
Data are number (%). p value tests the null hypothesis of equal proportions across study groups.
Table 3: Pathological tum our response
Primary end-point:ypCR
Articles
962 www.thelancet.com/oncology Vol 16 August 2015
during chemoradiation did not diff er between study groups (data not shown).
The proportion of patients experiencing adverse events during mFOLFOX6 treatment increased from group 2 to group 4. In group 2, two (3%) of 67 patients had grade 3 adverse events and one (1%) had a grade 4 adverse event; in group 3, 12 (18%) of 67 patients had grade 3 adverse events; in group 4, 18 (28%) of 65 patients had grade 3 adverse events and fi ve (8%) had grade 4 adverse events. The most common grade 3 or higher adverse events from mFOLFOX6 across study groups 2−4 were neutropenia in 11 patients (6%; fi ve in group 3 and six in group 4) and lymphopenia in seven patients (4%; three in group 3 and four in group 4). 18 (9%) of patients experienced neuropathy during mFOLFOX6 treatment, all of which were either grade 1 or 2. One patient had grade 1 neuropathy in group 2, six had grade 1 and one had grade 2 neuropathy in group 3, and nine had grade 1 and one had grade 2 neuropathy in group 4.
Table 2 summarises surgical results. The proportion of patients who received a sphincter-saving surgery and resection with negative margins was not signifi cantly diff erent between study groups (p=0·68 and p=0·089, respectively). The number of nodes examined and estimated blood loss were similar across all study groups (p=0·20 and p=0·62, respectively). Pelvic fi brosis, as measured by surgeon scoring from 1 (none) to 10 (maximum), increased in groups 2–4 (p=0·0001). However, the technical diffi culty of the operation, as
scored by the surgeon, was not signifi cantly diff erent across study groups (p=0·80).
No patient died during or after surgery in any study group. There was no signifi cant diff erence in the number of grade 3 or worse complications across study groups (all p>0·1; table 4). Grade 3 or worse complications were noted for nine (15%) patients in group 1, four (6%) patients in group 2, three (4%) patients in group 3, and six (9%) patients in group 4, when counting the maximum Clavien-Dindo grade complication for each patient. Of the 25 grade 3 or worse complications reported across all study groups, the most common were pelvic abscesses (seven patients: three in group 1, two in group 3, and two in group 4) and anastomotic leaks (seven patients: three in group 1, one in group 2, one in group 3, and two in group 4).
Using univariable logistic regression, we assessed known clinically relevant variables and study groups and their association with pathological complete response. The comparison of group 4 (the most intense regimen) with group 1 (the standard neoadjuvant regimen) showed a signifi cant association with pathological complete response (p=0·028; table 5). We then used multivariable logistic regression to model the probability of pathological complete response, examining whether a treatment eff ect on pathological complete response was present after adjusting for other known clinically relevant variables. In an intention-to-treat analysis that included study group, radiation dose, tumour stage, size, and distance to anal verge as variables, we found study group to be the only signifi cant predictor of pathological complete response (p=0·048; table 5). Patients in group 4 were signifi cantly more likely to achieve a pathological complete response than were patients in group 1 (odds ratio 3·49, 95% CI 1·39–8·75; p=0·011). In preplanned analyses, we tested two additional models in which we substituted study group for the treatment delivered as measured by cycles of mFOLFOX6 or the chemo radiation-to-surgery interval; both were signifi cant predictors of pathological complete response (p=0·028 and p=0·018, respectively).
Group 1 (n=60) Group 2 (n=67) Group 3 (n=67) Group 4 (n=65) p value
Time from start of chemoradiation to surgery (weeks) 14·2 (4·3) 17·1 (2·9) 21·0 (2·7) 25·2 (4·0) 0·0001
Time from end of chemoradiation to surgery (weeks) 8·5 (4·2) 11·1 (2·9) 15·4 (2·6) 19·3 (4·2) 0·0001
Sphincter-saving surgery 46 (77%) 50 (75%) 50 (75%) 44 (68%) 0·68
Ileostomy 38/46 (83%) 43/50 (86%) 47/50 (94%) 38/43 (88%)* 0·33
Resection with negative margins 59 (98%) 67 (100%) 64 (96%) 64 (100%)† 0·089
Number of nodes examined 12 (2–31) 14 (2–30) 13 (2–30) 11 (1–47) 0·20
Pelvic fi brosis‡ 2·4 (1·7) 3·9 (2·6) 4·4 (2·4) 3·9 (2·4) 0·0001
Technical diffi culty§ 4·6 (2·7) 4·9 (2·8) 5·1 (2·5) 4·8 (2·4) 0·80
Estimated blood loss (mL) 200 (50–1200) 225 (25–1500) 200 (50–1000) 150 (0–1000) 0·62
Data are mean (SD), number (%), n/N (%), or median (range). p values test the null hypothesis of equal means or proportions across study groups. *Information on whether an ileostomy was created or not was not available for one patient. †Data missing for one patient. ‡Scale ranges from 1 (none) to 10 (maximum). §Scale ranges from 1 (easy) to 10 (diffi cult).
Table 2: Surgical results
Group 1 (n=60)
Group 2 (n=67)
Group 3 (n=67)
Group 4 (n=65)
p value
Pathological complete response 11 (18%) 17 (25%) 20 (30%) 25 (38%) 0·0036
Partial response 44 (73%) 50 (75%) 46 (69%) 39 (60%) ··
Stable disease 5 (8%) 0 1 (1%) 1 (2%) ··
Data are number (%). p value tests the null hypothesis of equal proportions across study groups.
Table 3: Pathological tum our response

Timeeffect?PooledanalysisofRCT:Accord12/0405
EORTC22921
FFCD9203
CAO/ARO/AIO-94
CAO-ARO-AIO-04
INTERACTandTROG01.04
5247ptsà 3078à 440(14%)hadpCR
MedianSurgeryTimeà 6wks
Shorter Interval
Group< 6wks (1953pts)
Longer Interval
Group>6wks (1125pts)
p
pCR 11% 19% .01
ValentiniVECCO2017

Plateau @ 16 months
95% @ 11 months
Timeeffect?
ValentiniVECCO2017

Plateau @ 16 months
95% @ 11 months
Timeeffect?
ValentiniVECCO2017

Timeeffect?
• Retrospective pooled analysis 2094pts from21Italian Centers
• Median timetosurgeryà 9weeks• CumulativepCRà 22.3%
< 6wks 7-12wks > 13wks p
pCR
AIRO
12.6% 23.0% 31.1% <0.001
pCRGarcia- Aguilar
18%(8.1wks) 25%(11wks) 30%(15wks) 0.036
MacchiaGetal.onbehalf ofAIRO-GIsubmitted

pCR andOrgan Preservation
• Themeaning ofCR• Howtoachieve CR• What toirradiate
• Howtopredict

GTV + marginGTV + corresp mesorectum
Myerson et al IJROBP 2009
What toirradiate
3DConformal RT IMRT-IGRT

Imaging modality forGTV
(Pearson 0.24, p > 0.27) (Fig. 5). On T2, per ml volume increase theCI increased by 0.001 (SE 0.000, R2 0.367, p < 0.002) with interceptat 0.61. For Combi, per ml volume increase the CI increased by0.002 (SE 0.000, R2 0.371, p < 0.002) with intercept at 0.61.
Mean dCOMs per modality are presented in Fig. 6. T2 showed tohave the largest mean dCOM (3.30 mm) and Combi the lowest(2.57 mm), indicating T2 had most shape and location variability.Mean dCOMs were not significantly different between modalities(p > 0.70). dCOM variance was 2 times larger on T2 than on DWIor Combi, although one outlier existed on DWI. In retrospect,observers agreed the least experienced observer had delineatedan artifact which results in an unrealistically high dCOM value.
Contours were separated the widest on at least one point con-sidering the largest mean HD on T2 (18.59 mm, max 31.41 mm,min 9.16 mm) compared to DWI (mean HD 14.42 mm, min4.03 mm, max 49.10 mm) (Fig. 6). DWI again showed the outliercase (49.10 mm). On Combi, mean HD was between T2 and DWIvalues (Fig. 6). Repeated measures ANOVA showed a significantdifference in HD between modalities (p < 0.006), with a significantmean HD difference of 4.80 mm between T2 and DWI (p < 0.04) buta non-significant difference between T2 and Combi (p > 0.06) orDWI and Combi (p > 0.61). Separation over the whole contour-border was also largest on T2 (MD = 1.80 mm) and smallest onDWI (MD = 1.49 mm). Considering our voxel size ofP2.0 !P 2.0 mm on DWI, an average MD smaller than one voxelexists between observers. Again, the outlier case showed an
increased MD on DWI (9.05 mm). There were no significant differ-ences between modalities in MD (p > 0.19).
Discussion
In this study, two MRI sequences and their combination (T2 andDWI) were used for GTV-delineation in locally advanced rectal can-cer by three observers with the purpose of identifying GTV-delineation consistency and separation between contours. This isthe first study to actually calculate distance parameters, providingcrucial information for development of MRI-guided GTV booststrategies complementary to standard chemoradiation which isdirected to the entire CTV. Agreement between GTV delineationsperformed by the different observers was good and was compara-ble between different MRI sequences. Our results are in concor-dance with results of a previous rectal cancer delineation study[16]. Namely, the best consistency was reached on DWI images[16]. New findings of our study are inter-observer agreementimprovement with larger volumes, and comparable agreementafter combined T2-DWI sequences compared to both sequencesalone. This last finding may be due to the fact that some observerstend to rely mostly on T2-images while others prefer to trust theDWI-images more. Combining both scans, however, can alsointroduce a new inconsistency-factor since images have to be co-registered and topography can be different between sequences.
Fig. 1. Representative example of delineations in one patient of three observers on T2w (A and C) and DWI (B and D) sequences in transverse (A and B) and sagittal (C and D)direction.
402 Inter-observer agreement of MRI-based tumor delineation for preoperative radiotherapy boost in locally advanced rectal cancer
24pts
3observer
GTVon:• T2w
• DWI
• Combi T2-DWI
Bonnetain F etal.Eur J Cancer 2016

Imaging modality forGTV
of radical surgery. When watchful waiting is applied, the same MRsequences may not only play a role in delineation of tumors, butmay also be used to select patients with a cCR. For this purposeMR sequences are being optimized and new sequences are beingdeveloped, such as functional (DWI, MR spectroscopy) and veryhigh-resolution 7Tesla. These modalities are currently also underinvestigation in our institution [17,25] in order to provide a com-prehensive (MR-based) watchful waiting strategy to patients inthe future.
MRI-based delineation in rectal cancer is relatively new.Nowadays there is only one study available that investigatedinter-observer agreement [16]. Unfortunately, this study did not
calculate CIs or Fleiss’ kappa’s as measures of inter-observer agree-ment, but only compared volumes between observers (as dis-cussed above). In comparison with prostate cancer studies ondelineation agreement, we showed a considerably higher confor-mity on multiparametric (0.61) [14], T2w (0.61) and DWI (0.51)[15] imaging. This encourages to use MR for GTV delineationinstead of CT, which is the current standard for CTV delineation.Delineation of GTV volumes on CT resulted in contradictory vol-umes when compared to MRI GTV volumes [11,13], which is mostlikely dependent on worse soft-tissue contrast on CT. Whereas CTis primarily used to define CTVs, CT-based CTV delineationshowed comparable conformity indexes to what we obtained in
A B C0
50
100
150
200
T2A B
C D
Observers
Volu
me
[ml]
A B C0
50
100
150
DWI
Observers
Volu
me
[ml]
A B C0
50
100
150
Combi
Observers
Volu
me
[ml]
T2 DWI Combi0
50
100
150
200
All volumes per modality
Modality
Volu
me
[ml]
N Mean SD Min Max
T2 72 63.16 33.39 9.38 153.40DWI 69 41.94 25.49 7.60 115.18Combi 69 54.63 29.54 10.12 131.68
Fig. 2. Volumes per modality per observer (A–C), and for all observers combined per modality (mean, SD) (D).
0 50 100 150-20
0
20
40
60
80
A BAbsolute T2 and DWI volumes
Average volume [ml]
Mea
ndi
ffere
nce
[ml]
0 50 100 1500
2
4
Ratio between DWI to T2 volumes
Average volume [ml]
Diffe
renc
era
tio
1
3
Fig. 3. Bland–Altman plots representing absolute (A) and relative volume differences (B) between contours delineated by three observers on T2 and DWI.
404 Inter-observer agreement of MRI-based tumor delineation for preoperative radiotherapy boost in locally advanced rectal cancer
this study for MR-based GTV delineation [26]. Although theCT-based study by Nijkamp et al. was not aimed to identifydelineation-uncertainties for boost purposes, their presented
uncertainty-level indicates to what extent uncertainty is acceptedin current routine clinical practice. Nevertheless, there are somestudies that boost entire CTVs based on these uncertainties. They
Fleiss' kappa per modality
Flei
ss'k
appa
T2 DWI Combi0.5
0.6
0.7
0.8
0.9
1.0
N Mean SD Min Max N Mean SD Min Max
T2 24 .699 .078 .55 .84 T2 24 .820 .054 .71 .91DWI 23 .707 .104 .38 .85 DWI 23 .824 .079 .55 .92Combi 23 .690 .074 .53 .81 Combi 23 .815 .052 .69 .90
Conformity index per modality
Conf
orm
ityIn
dex
T2 DWI Combi0.0
0.2
0.4
0.6
0.8
1.0
A B
Fig. 4. Conformity index (A) and Fleiss’ kappa (B) values per modality for three observers.
T2
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
DWI
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
Combi
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
Pearson p Reg. coeff. SE R2
0.606 <0.002 0.001 0.00 0.367
Pearson p Reg. coeff. SE R2
0.240 <0.27 - - -
Pearson p Reg. coeff. SE R2
0.609 <0.002 0.001 0.00 0.371
A B C
Fig. 5. Correlation between volumes and conformity indexes (IC’s) for T2 (A), DWI (B), and Combi (C) modalities.
dCOM per modality
Dist
ance
[mm
]
T2DWI
Combi-5
0
5
10
15
20
T2 DWI Combi0
20
40
60
HDmax per modality
Max
imal
dist
ance
[mm
]
T2 DWI Combi-5
0
5
10
Mean distance per modality
Mea
ndi
stan
ce[m
m]
Distance parameters (mm)
dCOM N Mean SD Min Max
T2 24 3.30 1.67 .79 6.83DWI 23 2.88 3.51 .63 17.74Combi 23 2.57 1.60 .77 5.39
Distance parameters (mm)
MD N Mean SD Min Max
T2 24 1.61 .57 .75 2.79DWI 23 1.26 1.75 .29 9.05Combi 23 1.48 .56 .73 2.66
Distance parameters (mm)
HDmax N Mean SD Min Max
T2 24 18.62 7.01 9.16 31.41DWI 23 13.82 9.06 4.03 49.10Combi 23 15.38 6.26 8.01 30.27
Distance parameters (mm)
HDmax N Mean SD Min Max
T2 24 18.59 7.00 9.16 31.41DWI 23 14.42 9.15 4.03 49.10Combi 23 15.42 6.24 8.01 30.27
Distance parameters (mm)
MD N Mean SD Min Max
T2 24 1.80 .60 .75 2.79DWI 23 1.49 1.80 .29 9.05Combi 23 1.68 .60 .73 2.66
A B C
Fig. 6. The distance between centers of mass (dCOM) (A), maximal Hausdorff distances (HDmax) (B), and mean distances (MD) (C) of contours within each observer-pair permodality.
J.P.M. Burbach et al. / Radiotherapy and Oncology 118 (2016) 399–407 405
this study for MR-based GTV delineation [26]. Although theCT-based study by Nijkamp et al. was not aimed to identifydelineation-uncertainties for boost purposes, their presented
uncertainty-level indicates to what extent uncertainty is acceptedin current routine clinical practice. Nevertheless, there are somestudies that boost entire CTVs based on these uncertainties. They
Fleiss' kappa per modality
Flei
ss'k
appa
T2 DWI Combi0.5
0.6
0.7
0.8
0.9
1.0
N Mean SD Min Max N Mean SD Min Max
T2 24 .699 .078 .55 .84 T2 24 .820 .054 .71 .91DWI 23 .707 .104 .38 .85 DWI 23 .824 .079 .55 .92Combi 23 .690 .074 .53 .81 Combi 23 .815 .052 .69 .90
Conformity index per modality
Conf
orm
ityIn
dex
T2 DWI Combi0.0
0.2
0.4
0.6
0.8
1.0
A B
Fig. 4. Conformity index (A) and Fleiss’ kappa (B) values per modality for three observers.
T2
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
DWI
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
Combi
Volume [ml]
Conf
orm
ityIn
dex
0 50 100 1500.0
0.2
0.4
0.6
0.8
1.0
Pearson p Reg. coeff. SE R2
0.606 <0.002 0.001 0.00 0.367
Pearson p Reg. coeff. SE R2
0.240 <0.27 - - -
Pearson p Reg. coeff. SE R2
0.609 <0.002 0.001 0.00 0.371
A B C
Fig. 5. Correlation between volumes and conformity indexes (IC’s) for T2 (A), DWI (B), and Combi (C) modalities.
dCOM per modality
Dist
ance
[mm
]
T2DWI
Combi-5
0
5
10
15
20
T2 DWI Combi0
20
40
60
HDmax per modality
Max
imal
dist
ance
[mm
]
T2 DWI Combi-5
0
5
10
Mean distance per modality
Mea
ndi
stan
ce[m
m]
Distance parameters (mm)
dCOM N Mean SD Min Max
T2 24 3.30 1.67 .79 6.83DWI 23 2.88 3.51 .63 17.74Combi 23 2.57 1.60 .77 5.39
Distance parameters (mm)
MD N Mean SD Min Max
T2 24 1.61 .57 .75 2.79DWI 23 1.26 1.75 .29 9.05Combi 23 1.48 .56 .73 2.66
Distance parameters (mm)
HDmax N Mean SD Min Max
T2 24 18.62 7.01 9.16 31.41DWI 23 13.82 9.06 4.03 49.10Combi 23 15.38 6.26 8.01 30.27
Distance parameters (mm)
HDmax N Mean SD Min Max
T2 24 18.59 7.00 9.16 31.41DWI 23 14.42 9.15 4.03 49.10Combi 23 15.42 6.24 8.01 30.27
Distance parameters (mm)
MD N Mean SD Min Max
T2 24 1.80 .60 .75 2.79DWI 23 1.49 1.80 .29 9.05Combi 23 1.68 .60 .73 2.66
A B C
Fig. 6. The distance between centers of mass (dCOM) (A), maximal Hausdorff distances (HDmax) (B), and mean distances (MD) (C) of contours within each observer-pair permodality.
J.P.M. Burbach et al. / Radiotherapy and Oncology 118 (2016) 399–407 405
volume overlapping margins
Bonnetain F etal.Eur J Cancer 2016

Target motion
Shape
variation Set-up
•Prone up to 0.24 cm
•left-right direction
•Supine < 0.1 cm
Nijkamp J et al. Radiother Oncol 2009

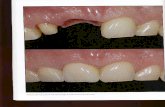
AFTERTREATMENTBEFORE
IMAGE GUIDED RADIOTHERAPY
MRI HYBRID Machines

pCR andOrgan Preservation
• Themeaning ofCR• Howtoachieve CR• What toirradiate• Howtopredict

CTPET
Nomograms forpCR
Van Stiphout R, Valntini V. et al. Radiother Oncol 2014
Van Stiphout R, Valentini V et al. Radiother Oncol 2011

Van Stiphout R, Valentini V et al. Radiother Oncol 2014
Nomogram
pCR (ypT0N0)
AUC = 0.70
highmediumlow

MRI ROIextraction FilterApplication
Dataanalysis(Moddicon):Modeldevelopment/validation
Radiomic inrectal cancer:
amodelforpCR prediction
Dinapoli N.et al.ESTRO35,Turin 2016
EXTERNAL
VALIDATION

Toremember
• Themeaning ofCR surg reduction• Howtoachieve CR RT;TIME• What toirradiate GTV-MR• Howtopredict nomograms