GnRH. Lormone che induce il rilascio delle gonadotropine (GnRH) è il principale regolatore della...
-
Upload
orabella-campana -
Category
Documents
-
view
214 -
download
0
Transcript of GnRH. Lormone che induce il rilascio delle gonadotropine (GnRH) è il principale regolatore della...

GnRH
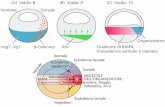
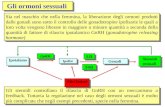
Lrsquoormone che induce il rilascio delle gonadotropine (GnRH) egrave il principale regolatore della cascata ormonale coivolta nella riproduzione ed egrave stato isolato dallrsquoarea preottica dellrsquo ipotalamo di mammiferi Ersquo un decapeptide ed egrave rilasciato nel circolo sanguigno a livello della eminenza mediana in modo pulsatile di quantitagrave precise dagli assoni di neuroni i cui terminali sono localizzati nellrsquoipofisi anteriore Il rilascio di questo ormone avviene ogni 30-120 minuti il quale regola la biosintesi e la liberazione di LH e FSH dallrsquoipofisi anteriore La frequenza di rilascio del GnRH egrave massima durante lrsquoovulazione mentre egrave ridotta al minimo durante la fase luteale La secrezione di GnRH ersquo controllata negativamente da steroidi gonadici mentre neurotrasmettitori ad azione inibitoria quali il GABA aminoacidi eccitatori (acido glutammico) peptidi oppioidi endogeni monoamine del sistema noradrenergico dopaminergico e serotoninergico acetilcolina melatonina ossitocina modulano il rilascio di GnRH
Sequenza di eventi causati dal GnRH
Ruolo degli aminoacidi nella sequenza del GnRH
GnRH agonisti
GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Lrsquoormone che induce il rilascio delle gonadotropine (GnRH) egrave il principale regolatore della cascata ormonale coivolta nella riproduzione ed egrave stato isolato dallrsquoarea preottica dellrsquo ipotalamo di mammiferi Ersquo un decapeptide ed egrave rilasciato nel circolo sanguigno a livello della eminenza mediana in modo pulsatile di quantitagrave precise dagli assoni di neuroni i cui terminali sono localizzati nellrsquoipofisi anteriore Il rilascio di questo ormone avviene ogni 30-120 minuti il quale regola la biosintesi e la liberazione di LH e FSH dallrsquoipofisi anteriore La frequenza di rilascio del GnRH egrave massima durante lrsquoovulazione mentre egrave ridotta al minimo durante la fase luteale La secrezione di GnRH ersquo controllata negativamente da steroidi gonadici mentre neurotrasmettitori ad azione inibitoria quali il GABA aminoacidi eccitatori (acido glutammico) peptidi oppioidi endogeni monoamine del sistema noradrenergico dopaminergico e serotoninergico acetilcolina melatonina ossitocina modulano il rilascio di GnRH
Sequenza di eventi causati dal GnRH
Ruolo degli aminoacidi nella sequenza del GnRH
GnRH agonisti
GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Sequenza di eventi causati dal GnRH
Ruolo degli aminoacidi nella sequenza del GnRH
GnRH agonisti
GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Ruolo degli aminoacidi nella sequenza del GnRH
GnRH agonisti
GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

GnRH agonisti
GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

GnRH antagonisti
Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Applicazioni cliniche dei GnRH agonisti e antagonisti
Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Background Systematic reviews have found that luteinizing hormone ndash releasing hormone (LHRH) agonists are effectivein treating premenopausal women with early breast cancerMethods We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In premenopausal Patients (ZIPP) which evaluated the LHRH agonist goserelin (36 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily) given for 2 years Women were randomly assigned to receive each therapy aloneboth or neither after primary therapy (surgery with or without radiotherapychemotherapy) Hazard ratiosand absolute risk differences were used to assess the effect of goserelin treatment on event-free survival(breast cancer recurrence new tumor or death) overall survival risk of recurrence of breast cancer andrisk of dying from breast cancer in the presence or absence of tamoxifenResults Fifteen years after the initiation of treatment for every 100 women not given tamoxifen there were 139 (95 confidence interval [CI] = 175 to 194) fewer events among those who were treated with goserelincompared with those who were not treated with goserelin However among women who did take tamoxifenthere were 28 fewer events (95 CI = 77 fewer to 20 more) per 100 women treated with goserelincompared with those not treated with goserelin The risk of dying from breast cancer was also reduced at15 years For every 100 women given goserelin the number of breast cancer deaths was lower by 26(95 CI = 66 fewer to 21 more) and 85 (95 CI = 22 to 137) in those who did and did not take tamoxifenrespectively although in the former group the difference was not statistically significantConclusions Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy In women who did not take tamoxifen there was a large benefit of goserelin treatment on survival and recurrence and in women who did take tamoxifen there was a marginal potential benefit onthese outcomes when goserelin was added
Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

Background The usefulness of long-term low-dose gonadotropin-releasing hormone agonist (GnRHa buserelin acetate) therapy so-called draw-back therapy for the treatment of adenomyosis was investigated MaterialMethods A retrospective observational study was conducted covering the period between January 2003 and March 2008 The subjects consisted of 12 patients with adenomyosis who underwent draw-back therapy for 2 years and had previously received GnRHa GnRHa was initiated at 900 μgday (6 nasalspraysday) When the CA-125 level normalized the GnRHa dosage was adjusted to 150ndash750 μgdayto achieve a plasma estradiol (E2) concentration of 20ndash50 pgml (ie the therapeutic window)Pain during withdrawal bleeding and chronic pelvic pain were assessed using a visual analoguescale In addition bone mineral density (BMD) of the lumbar vertebrae was measured using dualenergyX-ray absorptiometryResults The mean GnRHa dose during draw-back therapy was 435 μgday (29 nasal spraysday) The meanE2 level during draw-back therapy was 363plusmn143 pgml The intensity of chronic pelvic pain wassignifi cantly lower during draw-back therapy than before draw-back therapy and was nearly eliminatedin many patients (48plusmn12 vs 06plusmn07 respectively [p=0000]) Compared to the severity ofvasomotor symptoms during previous regular GnRHa therapy the severity of vasomotor symptomsduring draw-back therapy was signifi cantly lower (38plusmn07 vs 11plusmn07 respectively [p=0000]) Thedecrease in BMD during a 6-month course of treatment was 096plusmn09Conclusions GnRHa draw-back therapy allowed maintenance of plasma E2 levels within the therapeutic windowGnRHa can thus be administered for long periods of time while maintaining therapeutic effectson adenomyosis and suppressing adverse events
In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

In the long-acting GnRHa group (GroupL) patients received 36 mg of subcutaneous goserelin acetate (Zoladex1049299 depot AstraZeneca Ltd Kings Langley UK)In the short-acting GnRHa group (Group S) patients weresubcutaneously administered 05 mg of buserelin acetate(Superfact1049299 Hoechst AG Frankfurt Germany) or leuprolideacetate (Lucrin1049299 Labratories Abbot France St RemySur Avre France) daily at hospital before human chorionicgonadotrophin (hCG) administration
During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

During ovarian stimulation gonadotrophin-releasing hormone (GnRH) analogues are co-administered in order to prevent premature luteinizinghormone (LH) surges Premature LH surges are observedin about 20 of stimulated cycles without using GnRHanalogues [23] Avoiding the adverse effects of elevatedLH-levels first GnRH agonist analogues were used to supplementthe gonadotrophin stimulation The continuousadministration of GnRH agonists causes gonadotrophinsuppression through down-regulation and desensitizationof the GnRH receptors in the pituitary gland after aninitial short period of gonadotrophin hypersecretion [4]
In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

In this study we aim to evaluate the clinical outcome and patientrsquos convenience of single administration of long-acting GnRHa (goserelin depot) as comparedwith multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin)
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-

- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
-